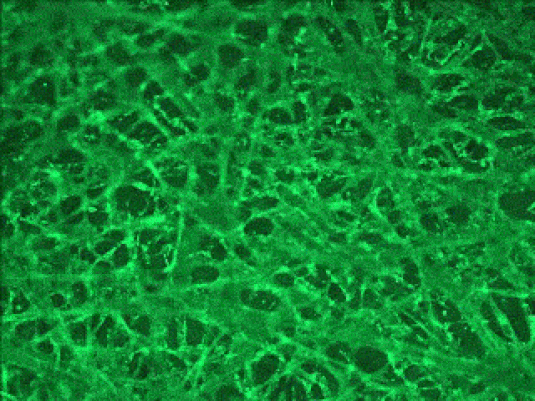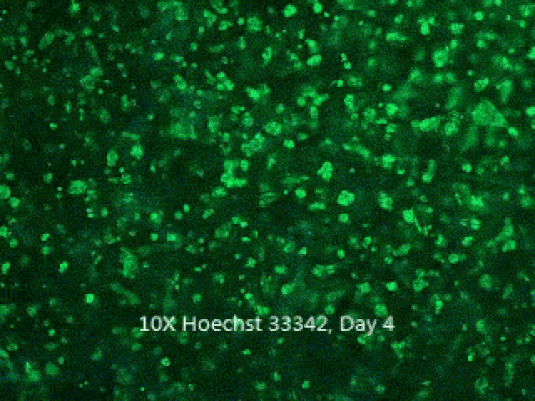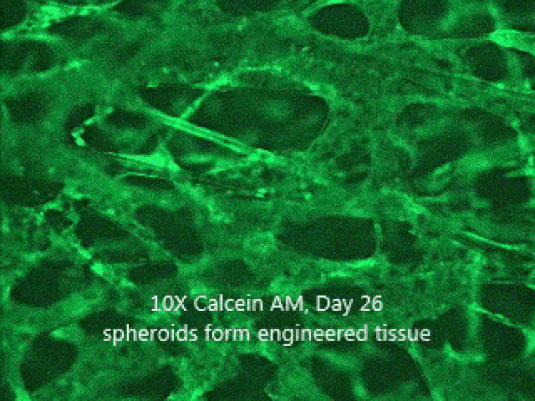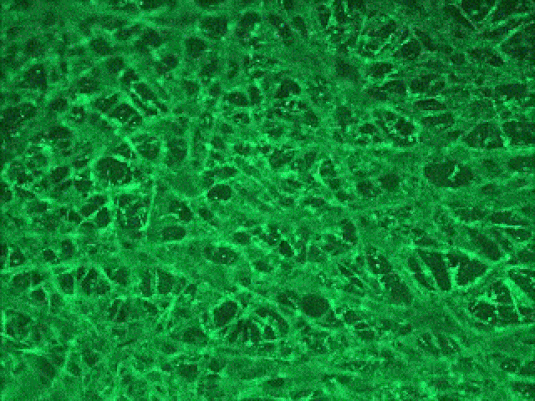
Human liver
|
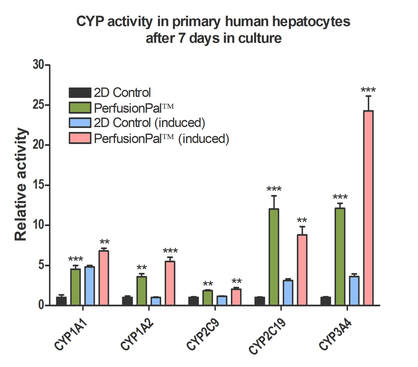
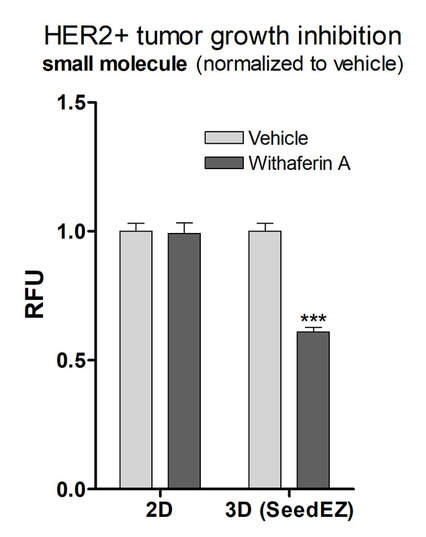
Increased CYP activity in Induced Primary Human Hepatocytes cultured with PerfusionPal
Phase 1 Drug Metabolism in primary human hepatocytes (BioIVT Cryoplateable 10-donor Liverpool) was measured over a 7 day period. Cells were cultured in 2D or PerfusionPal insert. At day 6, cells were induced with either Rifampin (10 uM) or Omeprazole (50 mM) and data was collected on day 7. Perfusion rate in PerfusionPal corresponds to 16 culture volume changes per 24 hour period. All values were normalized to the 2D control baseline CYP activity *p<0.05, **p<0.01, ***p<0.001.
|
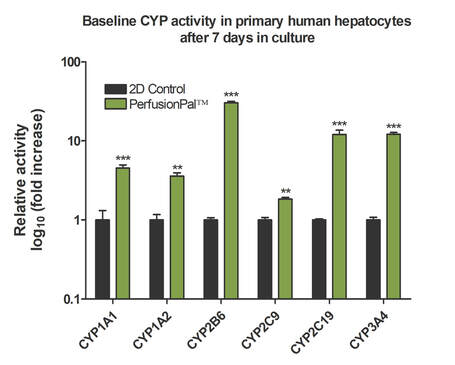
Baseline CYP Activity in Primary Human Hepatocytes after 7 days in culture
Baseline CYP450 activity was recorded for various CYP enzymes in primary human hepatocytes (BioIVT Cryoplateable 10-donor Liverpool) in cells cultured in 2D or PerfusionPal. Asterisk indicate significant difference in metabolic activity due to plating condition. Cells cultured in the Lena Biosciences PerfusionPal system show an enhanced CYP activity compared to the control. All values were normalized to the 2D control. Note: Log Scale for baseline activity graph. *p<0.05, **p<0.01, ***p<0.001.
|
Human brain
3D Cortical Tri-Culture Model
|
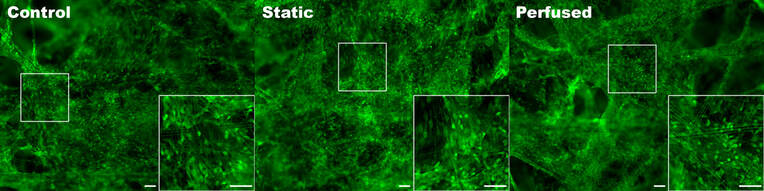
Triculture model of iPSC neurons, astrocytes and HMC3 microglia seeded in SeedEZ scaffolds and grown in one of 3 conditions. Scaffolds were stained using calcein AM.
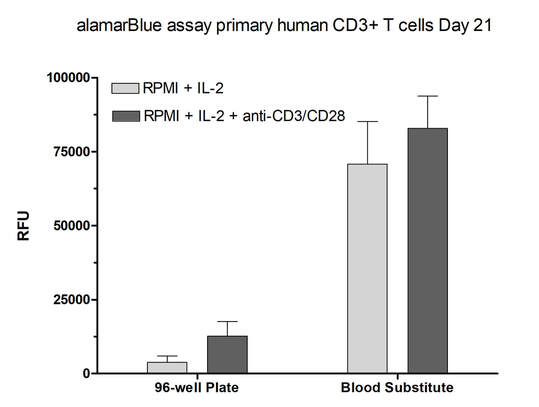
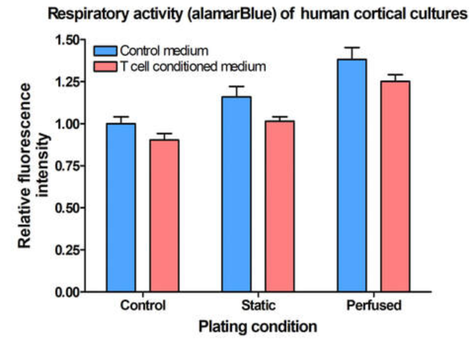
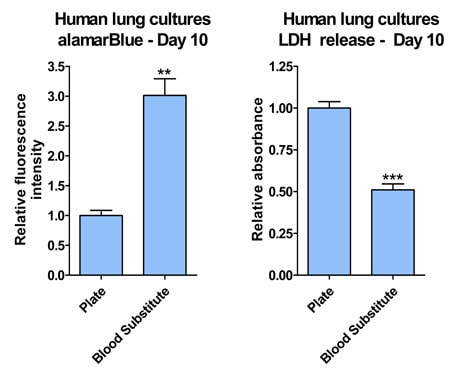
After 2 weeks in culture, media from iPSC neurons, iPSC astrocytes, and HMC3 microglia was sampled to detect off-target toxicity of activated T-cell medium using alamarBlue. Cells were grown in static conditions (blood substitute, without perfusion) or in Perfused conditions. Control conditions consisted of the triculture seeded in SeedEZ without blood substitute or perfusion.
|
Human lung cancer
|
Patient-derived non-small cell lung carcinoma (NSCLC) organoids cultured directly on the Blood Substitute (liquid-liquid interface)
Total cellular respiration on day 10, as measured by alamarBlue, showed that the cells on the Blood Substitute were three times more metabolically active than those in the multi-well plate. LDH release was twice as high in cells plated without Blood substitute, indicating that fewer cells died on the Blood Substitute than in the multi-well plate. **p<0.01, ***p<0.001 |